G6PD diagnostics
Importance of G6PD diagnosis for P. vivax treatment
The importance of being able to determine the G6PD status of a patient with P. vivax has risen over recent years with increasing awareness of the contribution of relapse to disease burden. Other reasons for determining G6PD status include an awareness of the risk associated with G6PD deficiency and primaquine therapy, and potential availability of the single-dose cure for P. vivax, tafenoquine. WHO recommends G6PD testing prior to primaquine administration mainly because haemolytic adverse events with primaquine were rarely noted in the literature. For tafenoquine, quantitative G6PD testing prior to its administration is required.
Diagnostics to exclude G6PD-deficient individuals mitigate the risk posed by therapy with primaquine or tafenoquine for P. vivax radical cure. The risk of haemolysis depends on the prevalence and severity of G6PD in the specific population as well as the dose of primaquine or tafenoquine. Individual risk also depends on whether the patient is already anaemic and the availability of clinical monitoring and, if necessary, blood transfusion services.
Need for new diagnostics and way forward
New diagnostics to detect G6PD deficiency are needed as the current ones are lacking in several areas. Qualitative tests which are commonly used do not accurately identify intermediate G6PD activities, often found in women, leading to possible severe health impacts as well as inaccurate or incomplete data on the prevalence of G6PD deficiency in women.1 See the section on G6PD deficiency in males and females for more information on this.
In addition, as a consequence of poor standardization across G6PD enzyme assay kits and the high sensibility of enzyme assays to conditions including salts, ph, and temperature, it is difficult to attribute laboratory-to-laboratory variation in normal G6PD values to differences in the population sampled. Current quantitative assays are also challenging to implement in clinical laboratories.
Access to G6PD testing should be expanded. Currently, testing is available only in some communities where there is known to be high prevalence of G6PD deficiency.
G6PD testing policy adoption
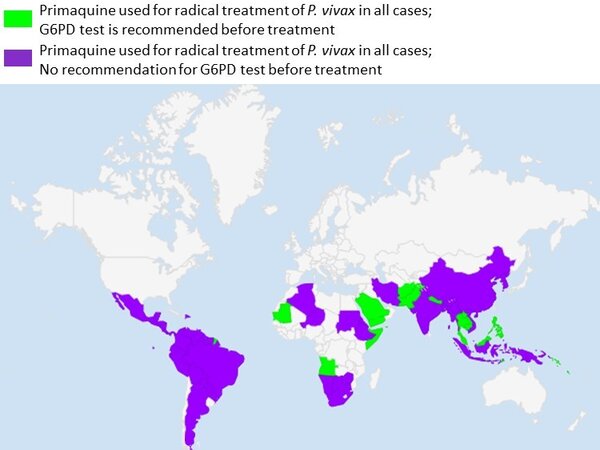
The WHO recommends G6PD testing before primaquine administration. However, policy adoption is not universal. G6PD testing will also be required before tafenoquine therapy.2
Below is a map showing where G6PD testing is recommended before treatment. Note that there may be differences in official recommendations vs use of the diagnostics in practice.
Regions where G6PD testing is recommended before treatment with primaquine
Requirements for future G6PD diagnostics
A target product profile (TPP) has been published describing the characteristics needed from improved G6PD diagnostics.3 Any new G6PD diagnostic will need to achieve WHO Prequalification to be adopted by National Malaria Programmes and included in the Global Fund list of approved diagnostics, so that countries are able to purchase them using Global Fund grants. Further details on the requirements for WHO prequalification may be found on this WHO page.